Avoiding Injections: Oral Biomacromolecule Delivery
Biotechnology Highlight: An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611-615. [1]
Laboratory: A recent report by the Langer lab at MIT describes the engineering and in vivo use of an oral delivery system for biomacromolecules using insulin as a model protein. Dr. Langer has received over 220 major awards in the fields of engineering and biotechnology, has written over 1,400 scientific articles, and has over 1,300 issued and pending patents worldwide. [2] He has trained top biomedical engineers and scientists that have established leadership positions around the globe, including UT Southwestern's Dr. Daniel Siegwart and Dr. Jinming Gao. [3]
Background and Overview: Biomacromolecules are large molecules present in biological systems ranging from endogenous and exogenous peptides, oligonucleotides, and carbohydrates that are used for indices such as diabetes, muscular dystrophies, and inflammation. [4] The major problems of biomacromolecule therapeutics revolve around their inability to be orally administered; their size, stability, and polarity make them insoluble in solutions, easily metabolized by the gastrointestinal tract, and poorly permeable through cellular tight junctions. [5] The Langer lab has developed biotechnology based on the self-righting leopard tortoise (S. pardalis) for oral delivery of biomacromolecules.
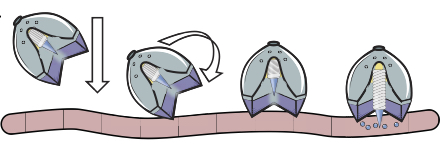
Summary of Results: As described in the text, the Langer lab has developed a device utilizing a self-orienting millimeter-scale applicator (SOMA) that when administered orally, self-orients in the stomach and injects a drug-loaded degradable post (millipost) into the stomach lining. The SOMA is encased in a polycaprolactone and stainless-steel bulb modeled after the self-orienting leopard tortoise (Stigmochelys pardalis). The shape of the device was modelled in MATLAB after the geometry of the tortoise shells and further optimized. 300 tests ex vivo inswine stomach and 60 tests in vivo in fasting swine displayed a combined 100% rate of correct orientation of the SOMA device, with a control only orienting correctly in 50% of the trials.

Fig. 1. [1]
With the orienting device prepared, the Langer lab researchers developed 7 mm long milliposts that would insert a compressed active pharmaceutical ingredient (API) into the lining of the stomach, utilizing insulin as their model biomacromolecule. The compressed formulation showed an increase in 300% shelf life and could be concentrated with up to 100 times more API than traditional liquid or solvent-based formulations. To ensure release of the millipost into the gastric lining, the researchers inserted a small 9-N steel spring into the SOMA that would propel the millipost. To control the spring release, a brittle sugar barrier was put in place that would be slowly hydrolyzed in the stomach, allowing the spring to drive the API-loaded millipost into the stomach lining.
The device was examined in vivo in swine during fasting, with an equivalent level of bioavailable insulin to traditional subdermal injection observed. Endoscopies were performed a week after administration of the SOMA, and no damage or irregularities were observed in the gastrointestinal tract. The researchers also performed a multi-device administration, delivering six SOMA prototypes to one pig simultaneously, and again saw no adverse effects. For further reading, see the full paper here.
Future Outlook: The technology described gives a new outlook on oral administration of biomacromolecules, a long-standing problem in the field of large molecule drug delivery. This represents one of the first devices to insert biomacromolecules in the stomach through the gastric lining with high bioavailability. Future directions include usage of other APIs such as other nucleic acid- and protein-based biotherapeutics, additional safety investigations, and examination of the properties of the API-containing millipost for drug delivery.
References:
1. An ingestible self-orienting system for oral delivery of macromolecules. Science 2019, 363, 611-615.
2. https://langer-lab.mit.edu/bio
3. https://siegwartlab.com/; https://www4.utsouthwestern.edu/gaolab/
4. Proteins: Recent advances in (therapeutic protein) drug development. F1000 Research 2017, 6, 113. Oligonucleotides: FDA-approved oligonucleotide therapies in 2017. Mol. Ther. 2017, 25, 1069-1075. Carbohydrates: Carbohydrates in therapeutics. Cardiovasc. Hematol. Agents Med. Chem. 2007, 5, 186-197.
5. Recent advances in oral delivery of biologics: nanomedicine and physical modes of delivery. Expert Opinion on Drug Delivery 2018, 15, 759-770.
Header image: source 1, Figure 1 A.