Checkpoint Inhibitor Market
What do these drugs do?
The immune system has the ability to differentiate between normal cells and "foreign" cells in part by using checkpoint proteins. Immune checkpoint proteins need to be activated or inactivated in order to trigger an immune response to attack cancer cells. Therefore, targeting immune system checkpoints such as PD-1, PD-L1, and CTLA-4 takes the brakes off of the immune system and helps T-cells destroy cancer cells.[1]How large is the immune checkpoint inhibitor market?
A recent market analysis published by Allied Market Research valued the global checkpoint inhibitor market at $10 billion in 2017.[2] The compound annual growth rate (CAGR) of 20.1% from 2018 to 2025 projects the market to reach $56 billion by 2025. The market report suggests that "technological advancements in screening procedure for cancer, increased healthcare expenditures, increase in government initiatives to alleviate cancer, and rise in public awareness are expected to boost the growth of the market. However, high cost associated with research activities is projected to restrain the market growth."[2] Shown to the left is another market analysis published in Nature Reviews- Drug Discovery which shows projected sales of leading checkpoint inhibitors for seven major markets.[3]
Which companies are leading the forefront in immune checkpoint inhibitor development?
According to analysis by Cision PR Newswire, "The leading companies of the global immune checkpoint inhibitors market include AstraZeneca PLC., Bristol-Myers Squibb Company, Eli Lilly and Company (ARMO Biosciences.), Fortress Biotech, Inc. (Checkpoint Therapeutics, Inc.), F. Hoffmann-La Roche Ltd. (Genentech, Inc.), Immutep Limited, Merck & Co., Inc., Merck KGaA (EMD Serono, Inc.), Novartis AG, and Pfizer Inc."[4] There are currently over 1,500 clinical trials ongoing for the use of these checkpoint inhibitors in a variety of cancer types.[5]
Checkpoint Inhibitors: Adverse Events
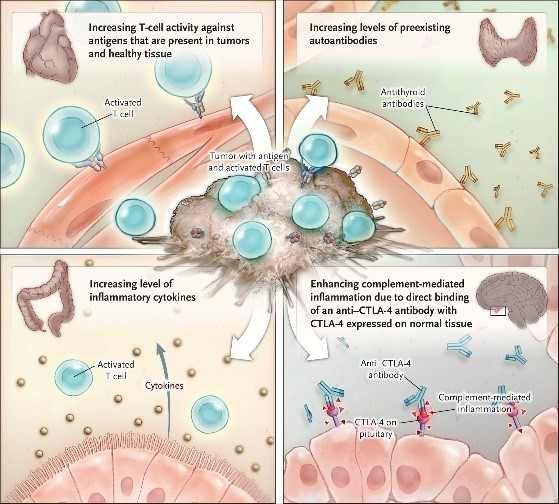
Checkpoint inhibitors are known to trigger adverse events in cancer patients by activating autoimmune responses. Possible mechanisms underlying immune-related adverse events are shown below. However, several studies suggest that the safety profile of these treatments are still better than chemotherapy.[6] The ability to identify patients at risk of adverse events will undoubtedly improve the safety profile of these drugs. A comprehensive review on immune-related adverse events was recently published by Postow et al in the New England Journal of Medicine.[6]
References
- https://www.cancer.gov/publications/dictionaries/cancer-terms/def/immune-checkpoint-inhibitor
- https://www.alliedmarketresearch.com/immune-check-point-inhibitors-market
- https://www.nature.com/articles/nrd4476#f1
- https://www.prnewswire.com/news-releases/immune-checkpoint-inhibitors-market-to-reach-56-53-bn-by-2025-allied-market-research-876099581.html
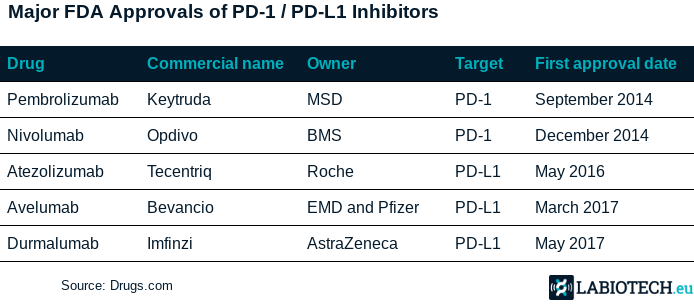
- https://labiotech.eu/features/pd-1-pd-l1-checkpoint-inhibitors/
- https://www.nejm.org/doi/full/10.1056/NEJMra1703481